Abstract

[Introduction]
CD19 chimeric antigen receptor (CAR) T cell therapies have been approved by the FDA for children and young adults with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) and adults with r/r large B-cell lymphoma. Recent reports about long-term follow-up of CD19 CAR T cell therapy in B-ALL (Maude et. al. NEJM 2018, Park et. al. NEJM 2018) suggest that the median event-free survival of children and young adult patients is longer than that of adult patients (Over 11 months versus 6.1 months). The reason for the difference between survival of pediatric and adult patient is unclear, but we hypothesize it is due to age-related changes in the T cells collected from patients. Therefore, we compared the function of CAR T cells derived from young or aged mice.
[Methods]
Young C57BL/6J (B6) mice (6-12 weeks) and aged B6 mice (³ 72 weeks) were used as donors for CAR T cell preparation. Four types of mouse specific CD19 CAR encoded GFP fusion proteins were evaluated with all having the same anti-CD19 scFv and CD8 hinge and transmembrane domains but differing in their intracellular domain (m19Δz: lacks the CD3ξ signaling domain, m19z: CD3ξ signaling domain only, m1928z: CD28 and CD3ξ signaling domains, m19-humBBz: 4-1BB and CD3ξ signaling domains).
[Results]
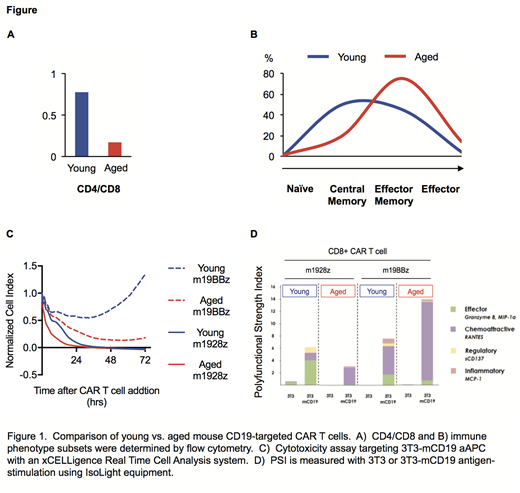
T cells isolated from the spleen of aged B6 mice were significantly fewer than those of young B6 mice. However, CAR transduction efficiency, viability and yield were similar between young and aged CAR T cells for each CAR group. All groups of aged CAR T cells predominate with CD8+ and effector-like phenotypes at the expense of CD4+ and memory-like phenotypes after CD19+ artificial antigen presenting cell (aAPC) stimulation (Fig. 1A-1B). Furthermore, compared to CAR T cells derived from young mice, aged CAR T cells (m19z, m1928z and m19BBz) exhibited superior cytotoxicity in a real-time cell analysis for CD19+ aAPC killing (Fig. 1C). Using our immune competent in vivo murine model, aged CAR T cells were short-lived and expanded poorly despite their superior in vitro cytotoxicity. To evaluate for potential mechanisms involving preferential production of effector-like CAR T cells from aged mice we performed gene-expression, as well as single cell secretory polyfunctional analyses. While the polyfunctional strength index (PSI) of CD8+ aged CAR T cells was higher for aged CAR T cells, the increased score was due mostly to abundant secretion of a chemokine (Fig. 1D). Furthermore, the RNA-DESeq analysis demonstrated increased expression of chemokines and perturbation of the EOMES/TBET transcription factor axis. RNA-DESeq also suggested that young CAR T cells were highly active in cell proliferation and cell differentiation whereas aged CAR T cells upregulated gene expression pathways that regulated responses to stimulus and exocytosis.
[Conclusions]
CAR T cells derived from aged mice exhibited enhanced cytotoxicity but shorter persistence and less memory-like phenotypes. Our results suggest that the difference of clinical outcome between younger patients and older patients may be due to an age-dependent CAR T cell phenotype that is reflected by its unique gene expression pattern, secretory profile, and/or transcription factor balance. In our future directions we are extending these observations to human CAR T cells and identifying potential methods to improve the function of aged CAR T cells.
Davila:Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal